Support
We are grateful for support from the United States Geological Survey (USGS) and Missouri Water Resource Research Center (MoWRRC) through Award Number G16AP00066, from the Great Rivers Cooperative Ecosystems Studies Unit through Award Numbers G21AC10041 and G21AC10446-00, from the National Science Foundation (NSF) Division of Chemical, Bioengineering, Environmental, & Transport Systems (CBET) Interfacial Engineering Program through Award Number 2131282 and Electrochemical Systems Program through Award Number 2219060, NSF Division of Civil, Mechanical, and Manufacturing Innovation (CMMI) Major Research Instrumentation Program through Award Number 2216026, NSF Division of Materials Research (DMR) Polymers Program through Award Number 2235161, NSF Division of Graduate Education NRT Program through Award Number 2243526 and from the American Chemical Society Petroleum Research Fund (ACS-PRF) through PRF# 65193-DNI5.
Techniques
Atomic Layer Deposition and Molecular Layer Deposition
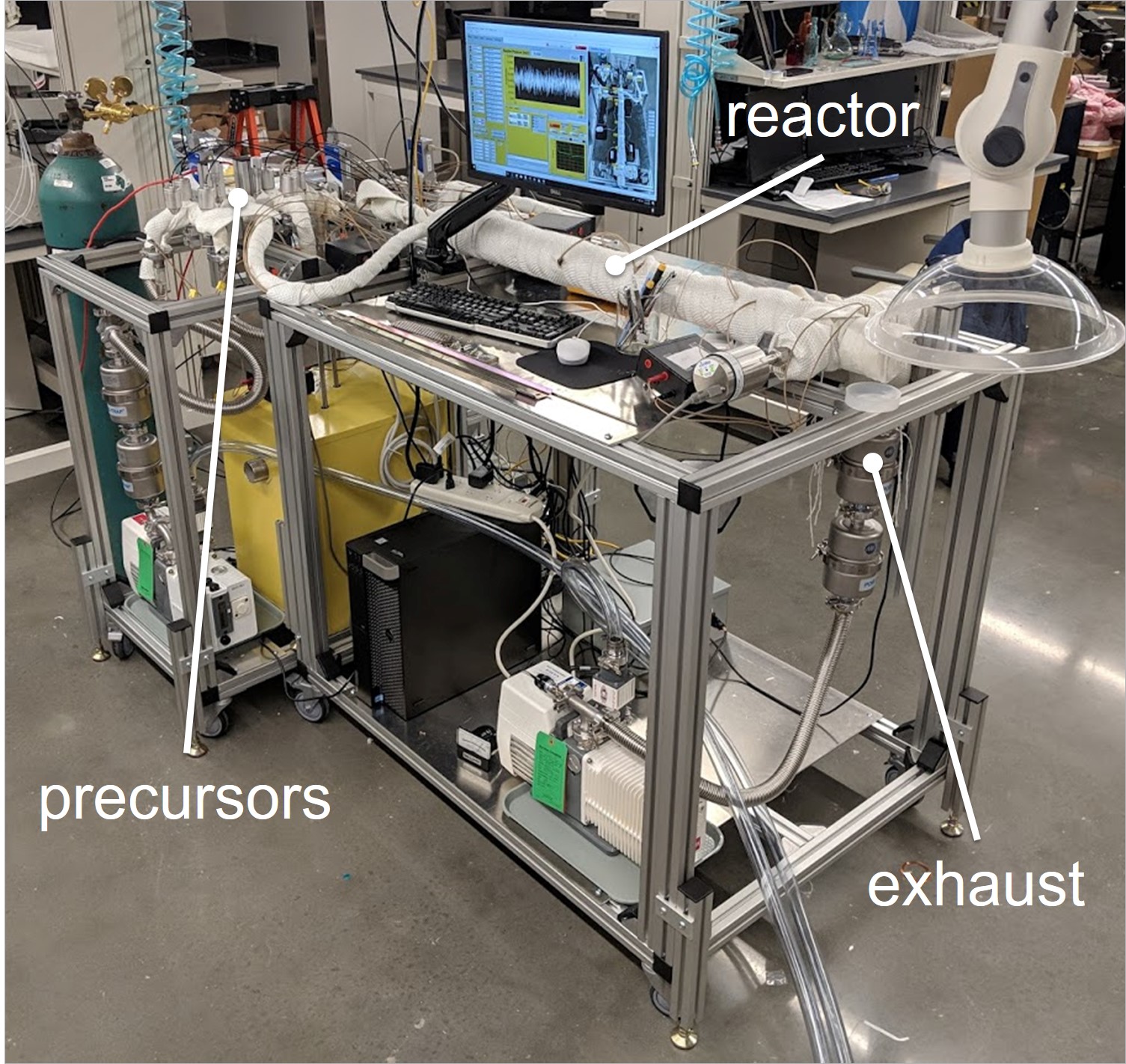
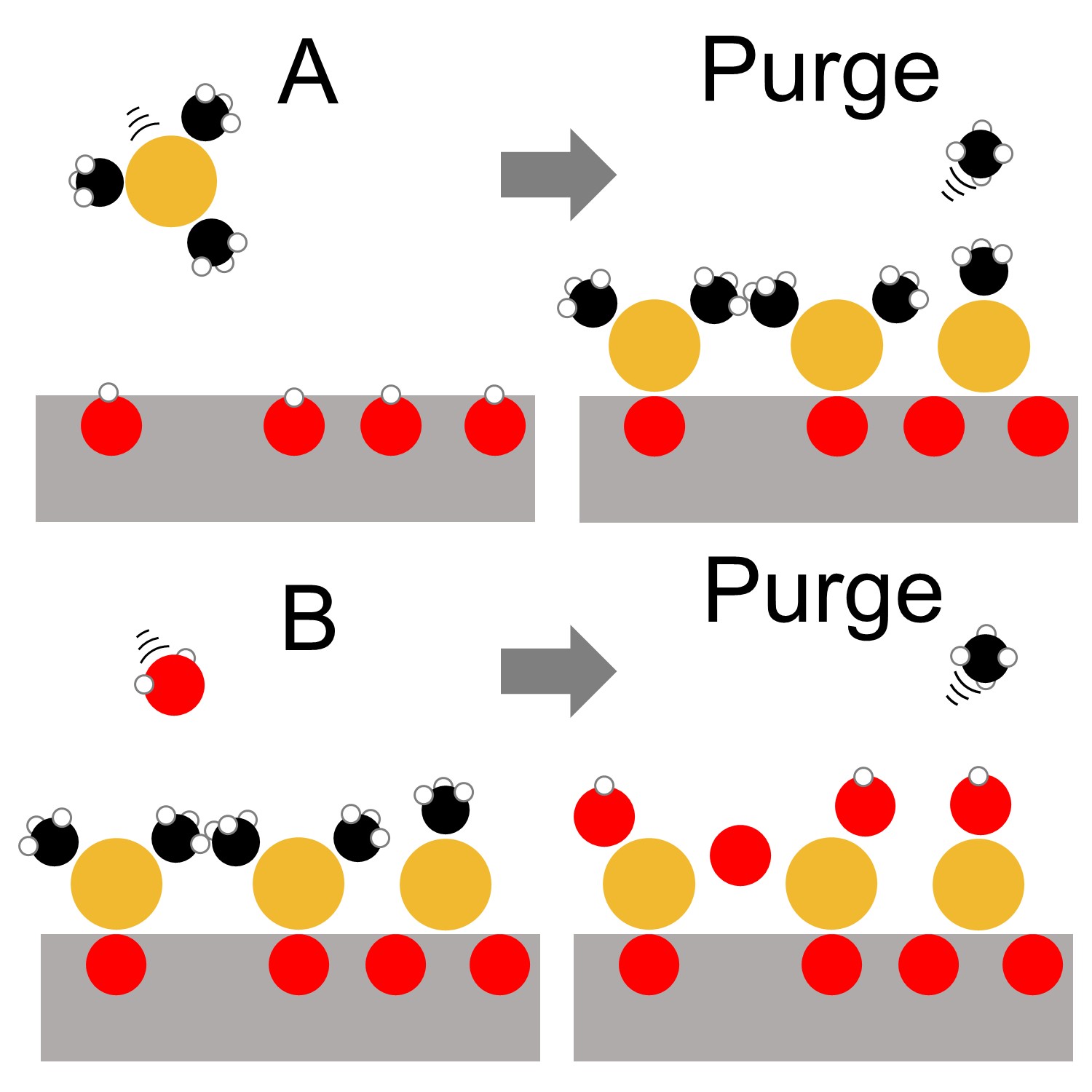
Our lab includes four customizable atomic layer deposition/molecular layer deposition (ALD/MLD) reactors, built and housed at the University of Missouri-Columbia. The reactor(s) are each equipped with gas channels for the simultaneous use of up to 7 precursors, and is expandable depending on the project needs. By using vapor-phase precursor exposures which undergo self-limiting surface reactions, we are able to deposit nanoscale thin films with atomic-scale control of thickness and composition. We accomodate substrates ranging from flat surfaces, to powders, to mechanical parts.
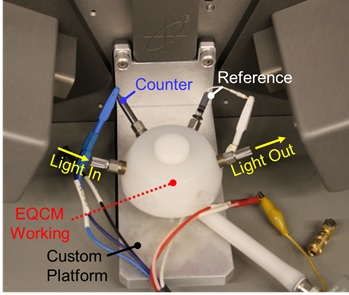
Electrochemical Characterization
Our lab is furnished with two potentiostats, both with EIS capability, and one with 1A, 48V capability, as well as two electrochemical quartz crystal microbalances (EQCM) with capacitive compensation. We also employ custom electrochemical cells for evaluation of flat samples, including in operando spectroscopic ellipsometry during EQCM. We are actively developing new electrochemical cells to understand degradation of electrode materials.
Projects
Molecular Assembly of Heteroatom-containing Redox-active Polymers
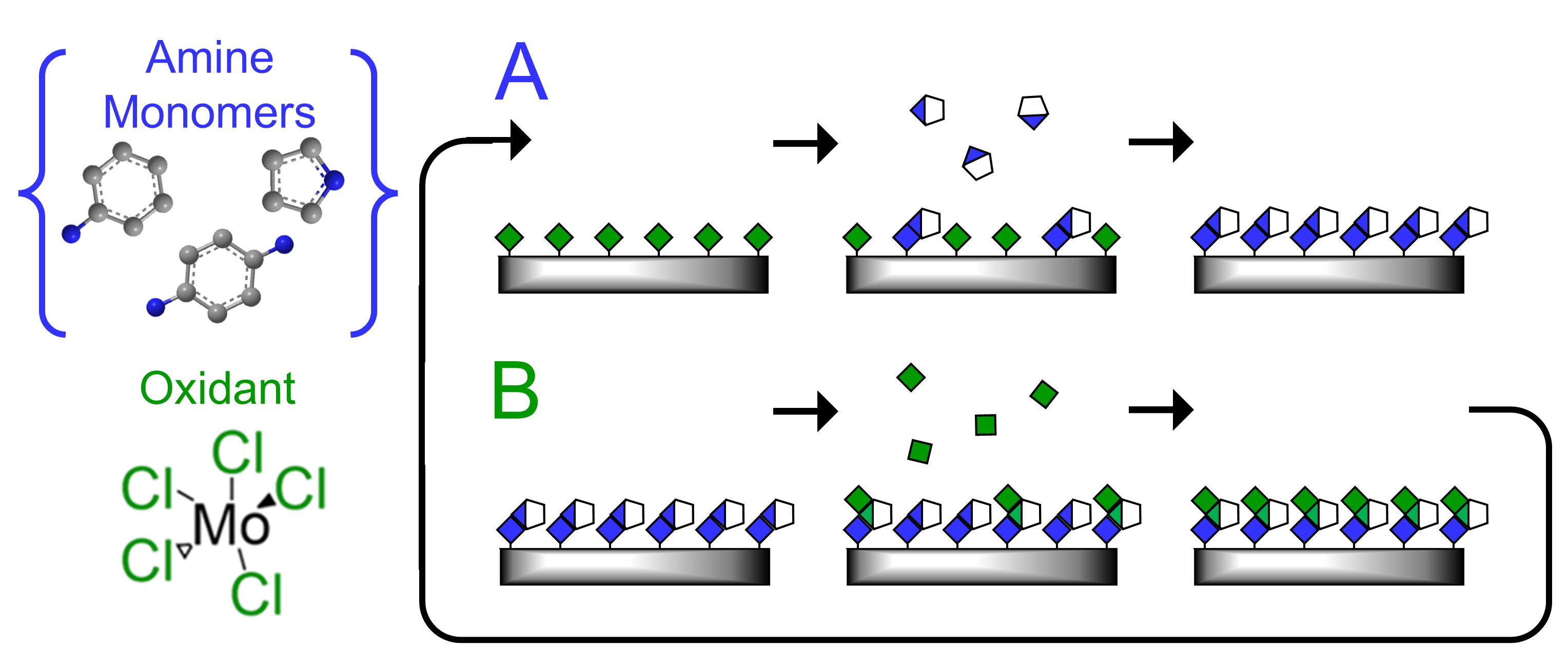
Electrically-conductive and redox-active polymers such as polyethylenedioxythiophene, polyaniline, and polypyrrole can be used for electrochemical water desalination, chemical sensing, and energy storage applications. However, conventional synthesis techniques for this class of polymers provide limited control over the local polymer structure. Our group is working to understand and control the electrochemical properties of these polymers through controlled molecular assembly. (See e.g. doi: 10.1021/acsapm.2c00942 and doi: 10.1021/acs.chemmater.2c02923) Moving forward, our focus is to leverage this precision synthesis to understand how the local molecular structure of these polymers impacts electrochemical properties including electron transport, ion transport, and redox-activity.
Local Atomic Structure Measurements using Pair Distribution Function Analysis
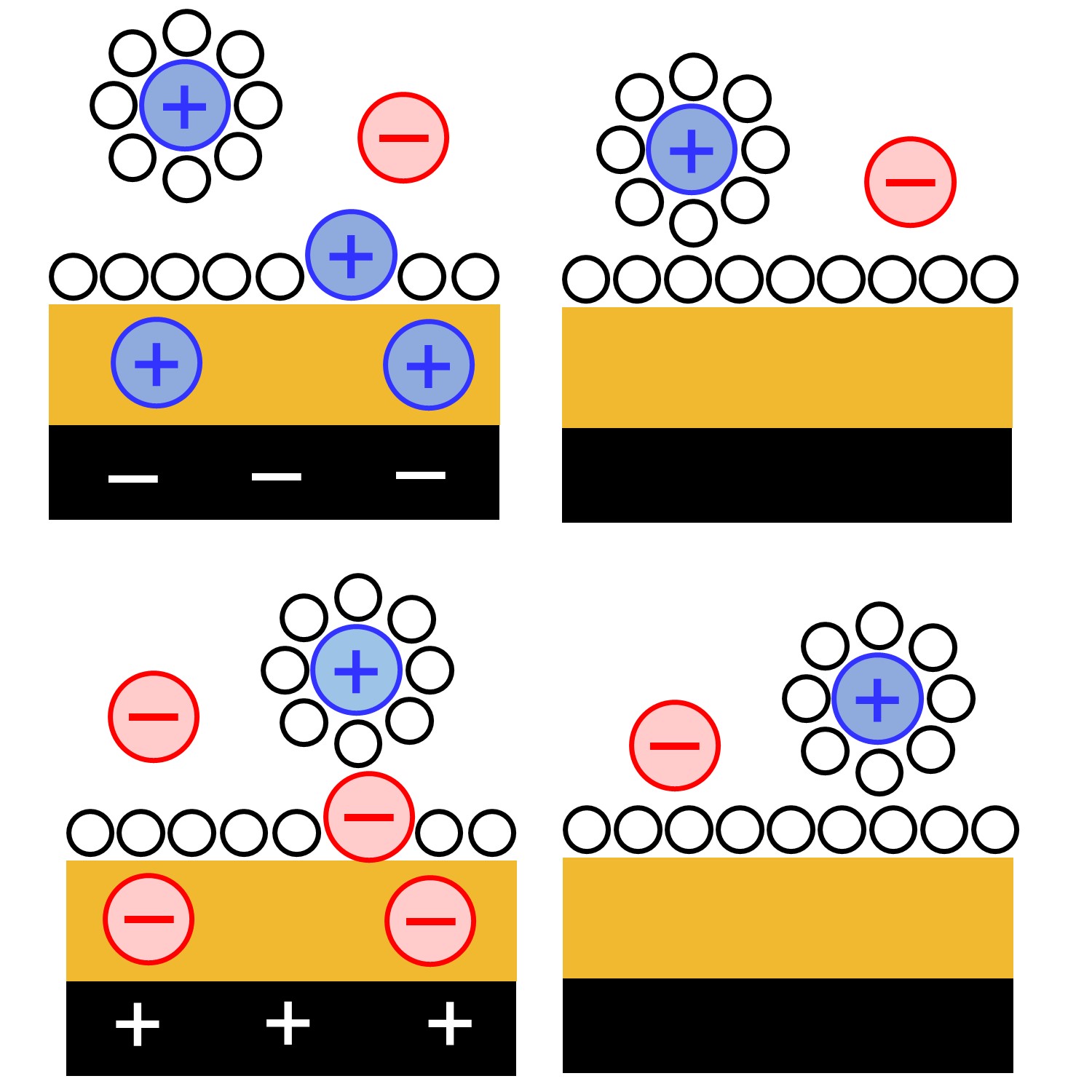
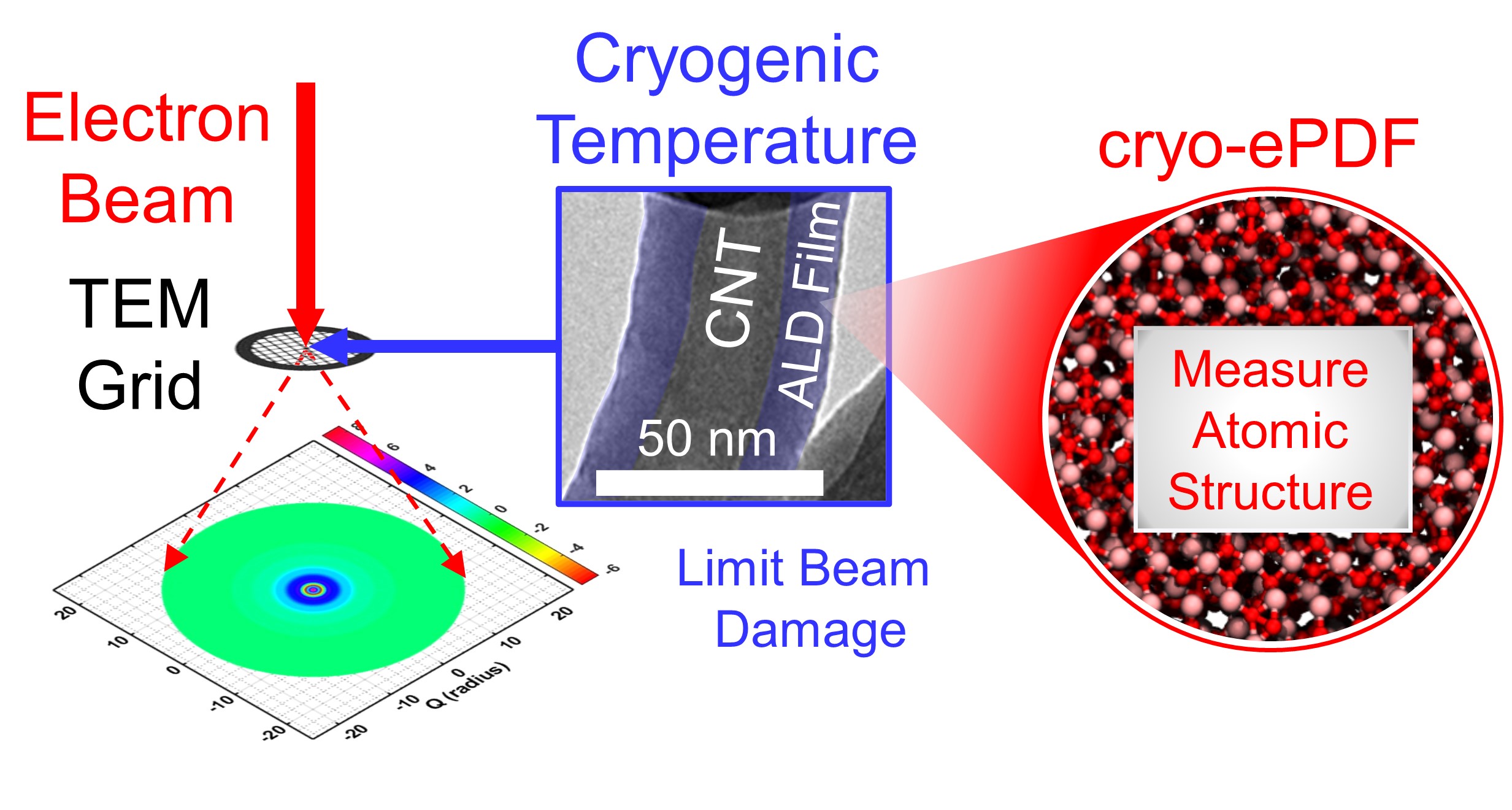
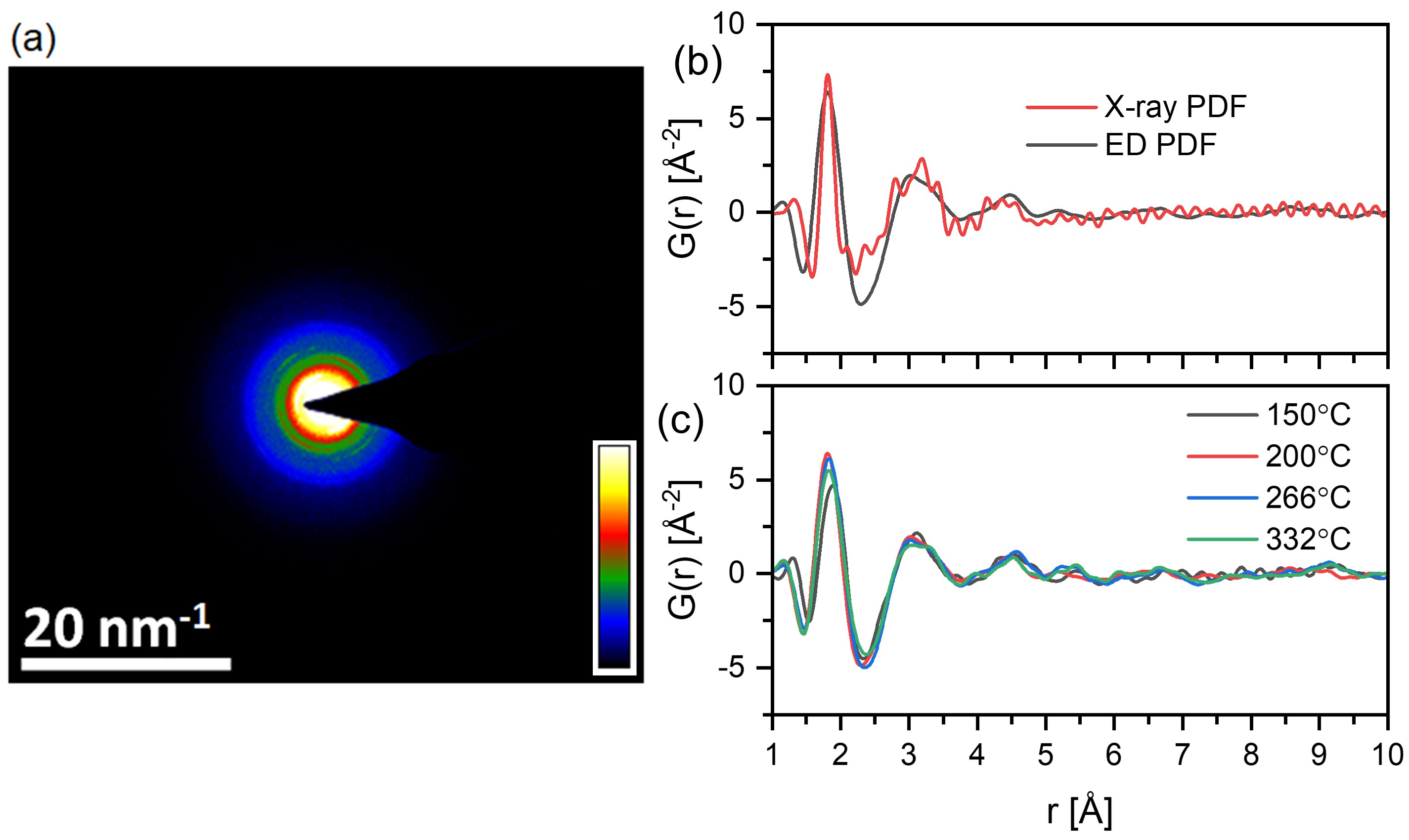
Measuring the atomic structure of amorphous interfaces promises to help us understand the behavior of materials in semiconductor devices, catalysts, batteries, and other applications. Our recent work has focused on understanding the atomic structure of amorphous materials deposited by atomic layer deposition (ALD) as model systems. Atomic layer deposition (ALD) provides uniform and conformal thin films that are of interest for a range of applications. Initial work demonstrated measurement of how the local atomic structure of ALD-grown aluminum oxide (AlOx) evolves in operando during growth by employing synchrotron high-energy X-ray diffraction (HE-XRD) (doi: 10.1021/acsami.0c01905) In more recent work, we report on efforts to employ electron diffraction pair distribution function (ePDF) measurements using more broadly available transmission electron microscope (TEM) instrumentation (doi: 10.1021/acsomega.0c06124 and doi: 10.1021/acsanm.2c02312). For more information on these efforts, see: From the Noise - Measuring the Atomic Structure in Amorphous Thin Films Grown By Atomic Layer Deposition - 6/18/2020.
Applied Machine Learning and Autonomous Experimentation
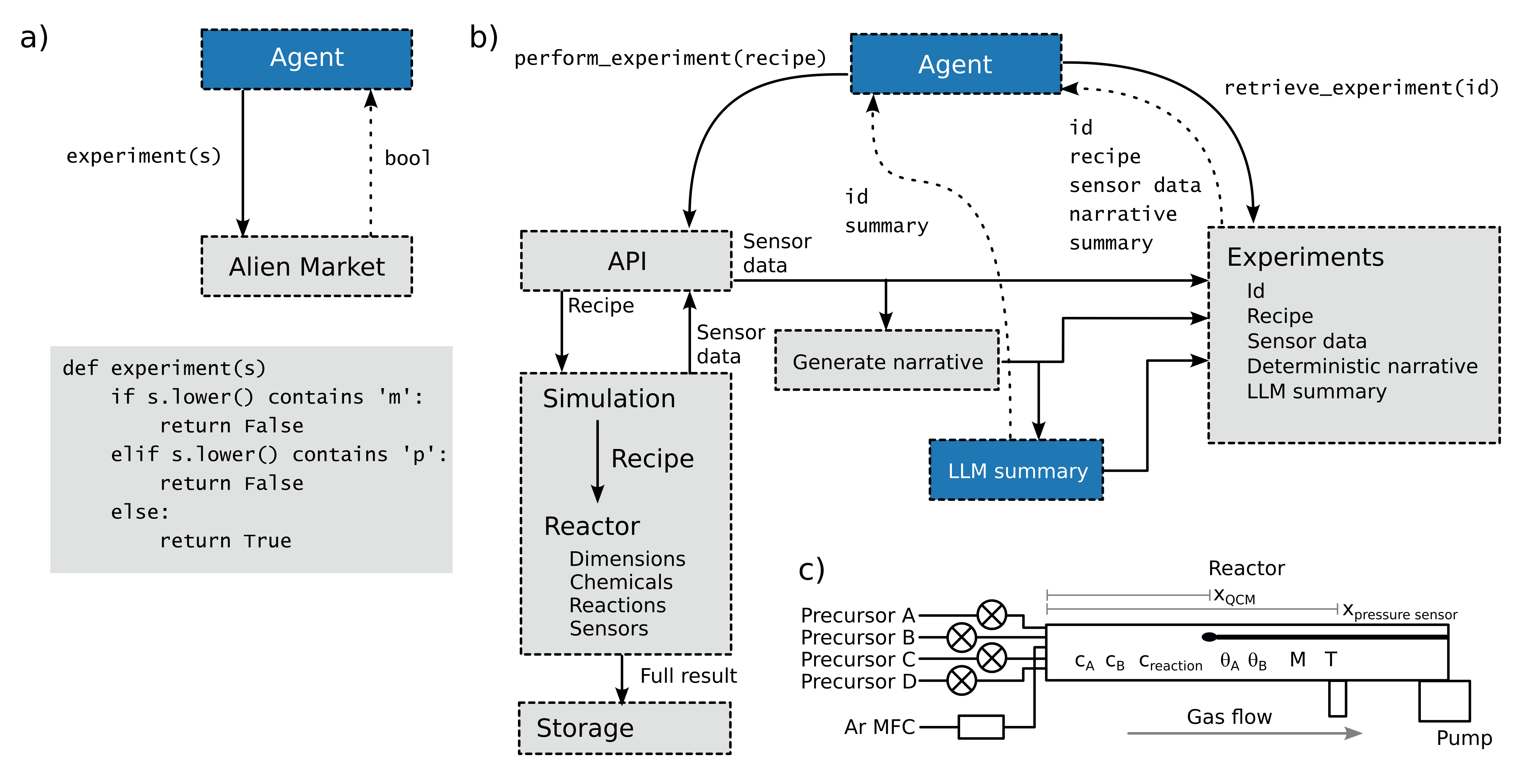
Our group is working to employ applied machine learning and data science approaches to accelerate materials discovery and understand structure-property relationships. Our vision is to leverage these tools to improve decision making and accerate the pace of materials innovation. We are actively working to build autonomous workflows for experimental materials science discovery with the goal of identifying new materials with emergent or previously unknown properties. As a requisite step towards "autonomation", automated experimental workflows are needed, which can require significant effort in less automated workflows. The ALD and MLD processes we employ are largely automated already, but do require effort for full autonomous integration that we are actively working to address. To date, we have applied machine learning and data science in various publications including the use of LLMs for experimental planning and chemical process discovery (doi: 10.48550/arXiv.2509.26201) multi-objective Pareto front optimization (doi: 10.48550/arXiv.2509.08988), randomized linear algebra and non-negative matrix factorization (doi: 10.48550/arXiv.2507.17068), and non-local averaging with employing acyclic graph workflow management (doi: 10.1002/aenm.202403904).
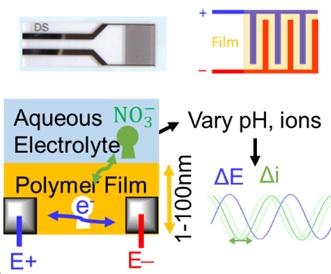
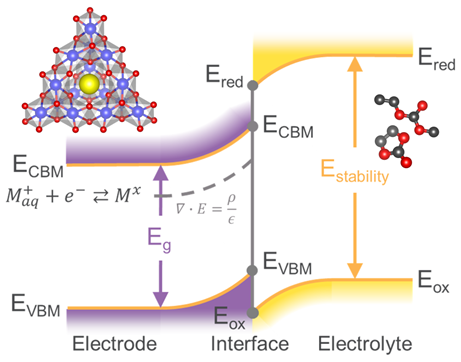
Nitrate Sensors from Polymer thin Films
Each year over $100 million worth of nitrogen fertilizer is lost to water runoff in Missouri and collects in Missouri water resources, causing harmful algal blooms that damage the environment and leading to economic losses to waterfront communities. In order to mitigate these harmful effects, we need to better understand how nitrogen from fertilizer is lost and transported in the environment. Conventional methods for detecting nitrogen in water or soil samples rely on slow and costly laboratory measurements, most commonly liquid chromatography-mass spectrometry (LC-MS) methods. The sparse data from these measurements cannot provide a clear picture of how nitrogen moves through the environment. We aim to develop low-cost and high accuracy nitrogen sensors based on electrically-conducting polymer thin films. The goal is to enable real-time distributed monitoring of nitrate in the environment in line with efforts such as EARTHDATA from NASA. These polymer films must be stable in the environment to be used in sensors. Recent work (doi: 10.1063/5.0035309) has established a multimodal tool to measure the degradation of polymers in real-time and inform changes to the polymers for improved stability. For more information on these efforts, see: MOWRRC Seminar - 4/30/2021.